Header Image: Pfizer
This seeks to summarize the report to the FDA by Pfizer on October 26, 2021 that led to its authorization for use in children age 5-11 in the United States. It is not meant to be a comprehensive review. All figures are directly from the study. Important findings are in bold, and significant findings are highlighted in yellow.
Background
Data from March to December 2020, before the appearance of the Delta variant:
| Age 0-4 | Age 5-10 | Age 11-13 | Age 14-17 | |
| Risk of Infection (1.64% overall) | 1.1% | 1.3% | 1.7% | 2.7% |
| Which Children are Infected | 17.4% | 25.7% | 18.6% | 38.3% |
| Symptomatic cases | Estimated range 16-35% | |||
| Who are Hospitalized due to Infection: | 36.1% | 16.7% | 13.5% | 33.7% |
| Who are Admitted to ICU : | 33.3% | 19.4% | 15.1% | 33.2% |
| Who Died: | 29.2% | 16.9% | 15.1% | 38.8% |
Data from March 2020 to September 2021, after the arrival of the Delta variant in summer 2021:
| Age 5-10 before Delta | Age 5-10 after Delta | Rate of Increase | |
| Risk of Infection | 1.27% | 7.3% | 5.7 times |
| Risk for Hospitalization | 0.008% | 0.035% | 4.4 times |
Transmission:
- Children are unlikely to be primary source of transmission in the household.
- Children under the age of 10 has lower transmission rate (5.3%) than older children (18.6% for age 10-19).
Infection:
- Children under 10 seems to be infected at the same rate as adults.
- However, they are more likely to have asymptomatic or mild cases than adults.
- They are sick for about 11 days on average (range of 1 to 36 days).
- Children can spread for up to 21 days after initial positive test results, even though they might not have any more symptoms.
Symptoms:
- Fever was most common
- Cough, runny nose, sore throat, headache
- Nausea, vomitting, diarrhea are less common
Risk factors for severe infection:
- Obesity
- Chronic respiratory diseases (such as asthma)
- Compromised or suppressed immune system
- Neurological conditions
- Congenital heart diseases
MIS-C, a rare (0.2%) but serious complication of COVID-19:
- Average age: 8-9 years
- 62% were male
- 60% were Hispanic or African-American
- 73% previously all healthy
- 80% required care in ICU (intensive care unit)
- 20% requires a ventilator
- 2% died
The Data
Total participants: 49
Demographics: 74.2% White, 12.9% Asian, 9.7% African-American, and 3.2% other racial groups. 6.5% was Hispanic/Latino was reported.
Median age: 9
Male was 48.4%, Female 51.6%
Dose tested: 10μg and 20μg, with similar response.
With the lower 10μg dose having less reaction, it was the chosen dose for phase 2/3 Trial.
The 30μg dose (the dose for age 12 and up) was too strong and not considered for Phase 2/3.
Total participants: 2285, with 757 receiving placebo, and 1528 receiving the vaccine.
Demographics: 78.9% White, 6% Asian, 6.5% African-American, and 7% multiracial, and <1% others. 21.1% Hispanic/Latino was reported.
Median age: 8
Male was 52.1%, Female 47.9%
Obese children: 11% of vaccine group, and 12.3% of placebo group.
20% with underlying illness: asthma, neurological disorders, and heart disease.
Both groups have similar rate of previous SARS-CoV-2 infection.
98.7% completed the study. Withdrawals were due to parent/guardian decisions, not side effects or reactions.
Follow-up time: more than 2 but less than 3 months after 2nd dose.
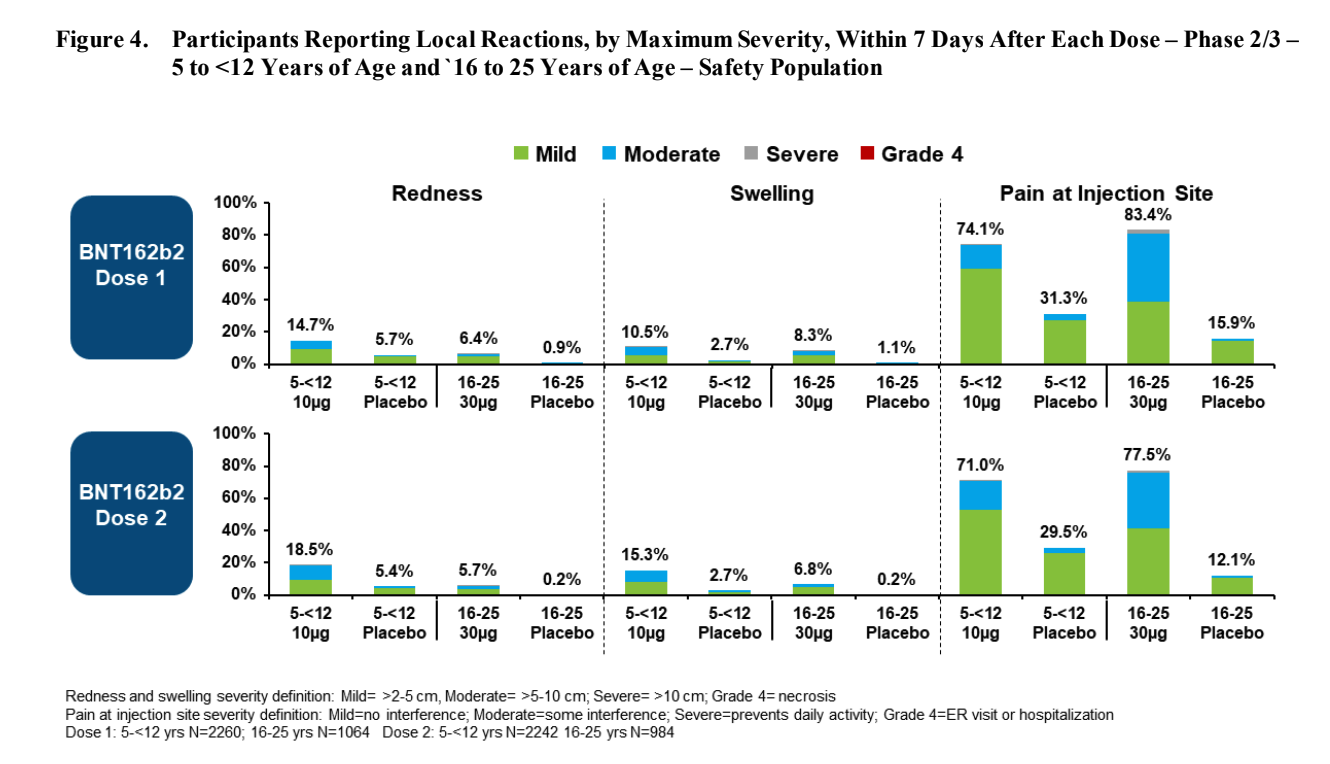
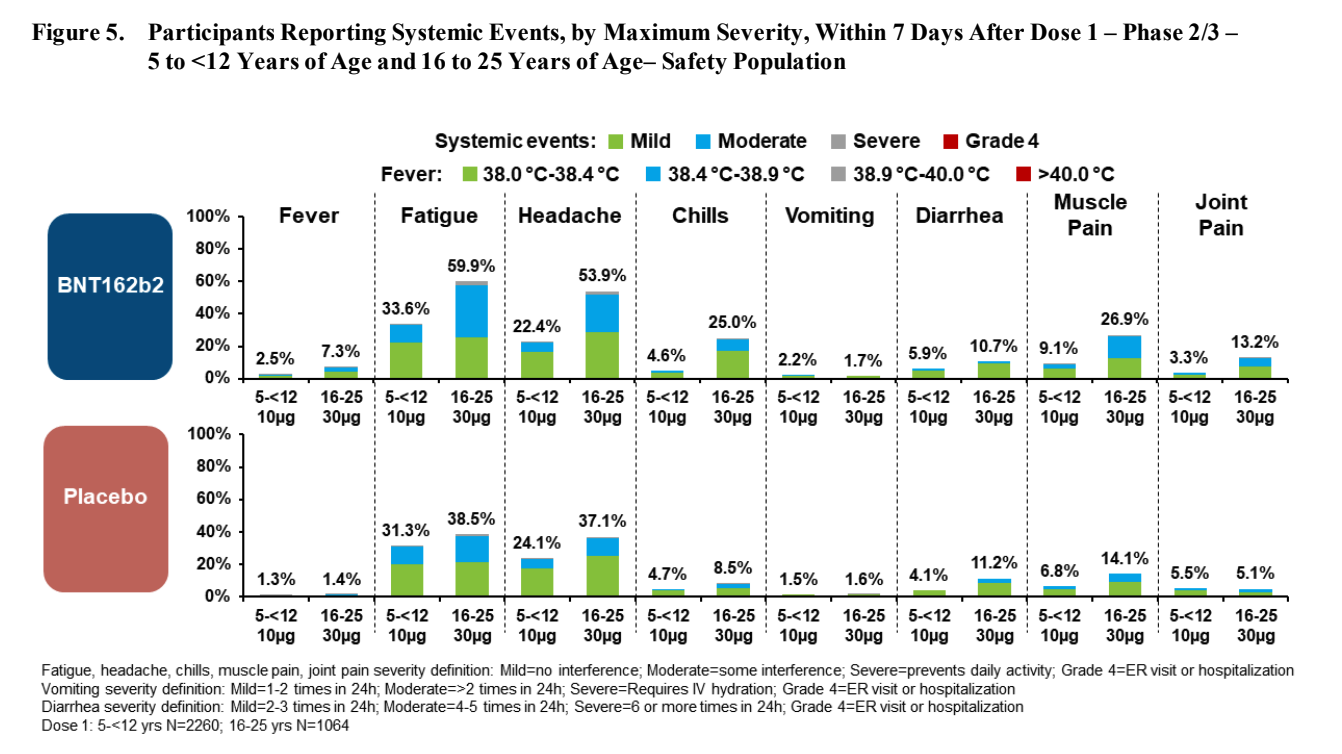
After the first dose, compared to the age 16-25 group, participants age 5-11 tends to have:
- More redness (twice as likely)
- About the same amount of swelling
- Slightly less pain at injection site
- Less fever (3 times less likely) or chills (5 times less likely)
- Less fatigue (half as likely)
- Less headache (half as likely)
- Slightly more vomiting (20% more), but less diarrhea (half as likely)
- Less muscle ache (2.5 times less likely) and joint pain (4 times less likely)
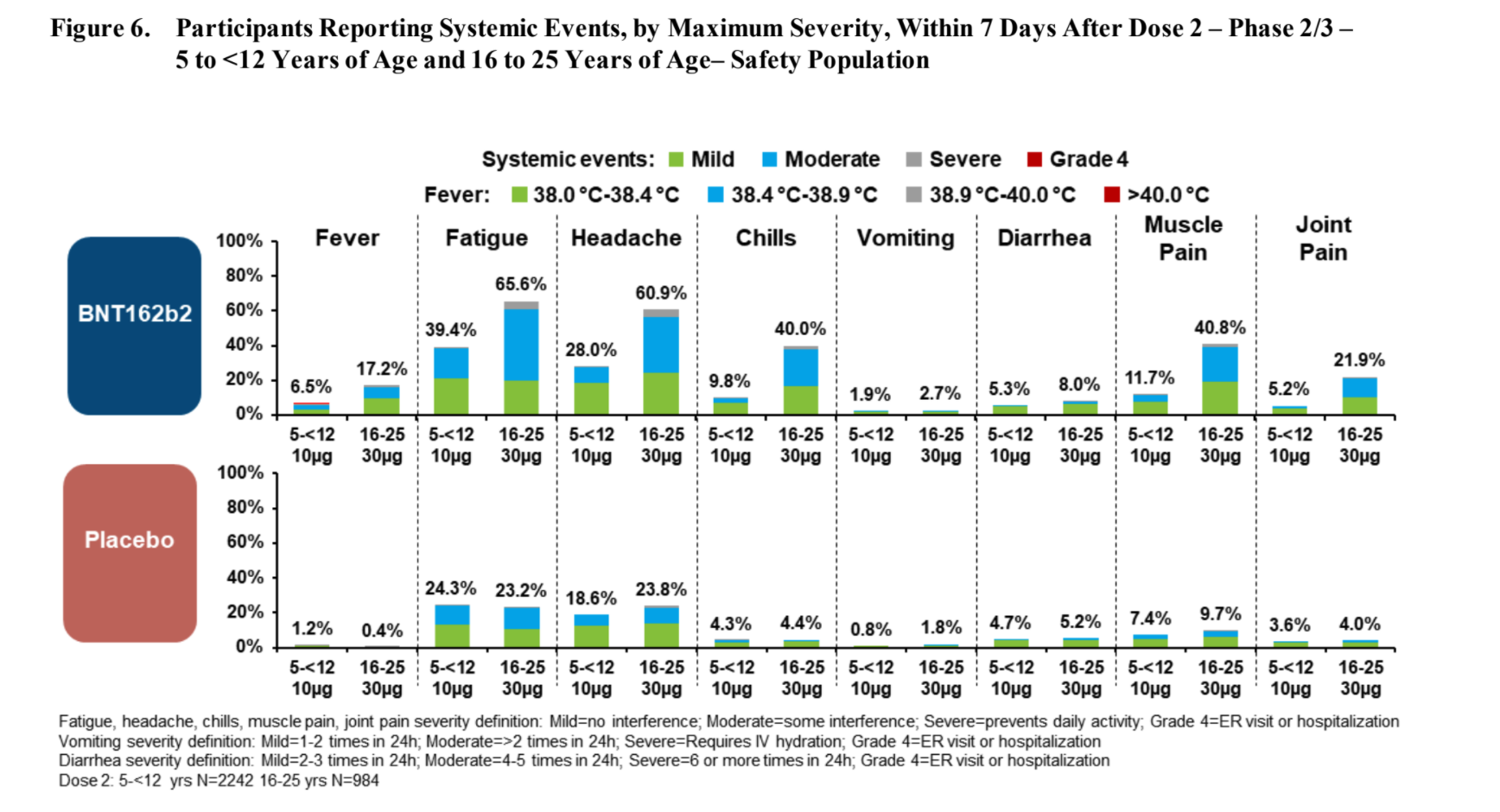
After the second dose, compared to the age 16-25 group, participants age 5-11 tends to have:
- More redness (3 times more likely)
- More swelling (2 times more likely)
- But slightly less pain at injection site, and
- Less fever (3 times less likely) or chills (4 times less likely)
- Less fatigue (half as likely)
- Less headache (3 times less likely)
- Less vomiting and less diarrhea
- Less muscle ache (3.5 times less likely) and joint pain (4 times less likely)
Reactions after 2nd Dose
Results
- No deaths were reported in either group.
- No severe COVID-19 cases or MIS-C were reported.
- No cases of anaphylaxis or anaphylactic/anaphylactoid reaction were reported.
- The vaccine works against both the original and the Delta variant and full vaccine effectiveness was achieved at 7 days after the 2nd dose.
No participants reported any serious adverse 1 month after dose 2.
1 participant reported a serious adverse event (not leading to withdrawal) from dose 1 to 2-3 months after dose 2.
Other events reported — overall the same or just minimally higher than the general children population:
| Reaction | Vaccine group | Placebo group |
| Infections and infestations | 1.9% | 2% |
| Injury or poisoning | 1.7% | 0.7% |
| Psychiatric disorders | 0.3% | 0.4% |
| Blood or lymph node disorders | 0.9% | 0.1% |
| Rash | 1.4% | 0.8% |
| Immue system disorders | 0.1% | 0.1% |
Other significant reactions:
- Joint pain: 1 participant of 1528, or 0.2%
- Numbness/tingling: 1 participant of 1528, or 0.2%, gone after 3 days
Breakthrough Infections: 10 times less likely
- 3 cases of COVID-19 in the participant group (0.2%):
- The 3 cases were at 50, 96 and 112 days after the 1st dose
- All 3 participants reported cough and sore throat
- 1 participant reported headache, runny nose, and nausea
- 16 cases of COVID-19 in the placebo group (2.1%):
- More than half reported fever, sore throat, runny nose, cough, and muscle pain
- 1 in 3 reported fatigue, chills
- 1 in 4 reported headache, and loss of taste and smell
- 1 in 5 reported nausea, abdominal pain, diarrhea
- 1 reported shortness of breath
Myocarditis (heart inflammation) is an important complication of COVID-19 in children. From a study in India, this occurred in 4% of children infected with SARS-CoV-2, of whom 78% died.
One can also get myocarditis as a reaction from the vaccine, but fortunately it is very rare, and almost always mild and temporary (as seen in the older children age group). Based on those data, it is extrapolated that the rate is 43 cases per 1 million vaccinated males (0.0043%, or 1,000 times less likely if vaccinated), and 4 per 1 million vaccinated females (0.0004%, or 10,000 times less likely if vaccinated). This will be more better known when more children are vaccinated.
Discussion
Based on all the combined data, children age 5-11 are:
- At moderately high risk of contracting COVID-19 overall since the Delta variant;
- Unlikely to die from COVID-19 (1.2 per 1 million pre Delta, no data availabe since Delta);
- 10 times less likely to have a more severe case of COVID-19 when vaccinated.
The vaccine has been shown to
- Be very safe when observed for up to 3 months (and ongoing since June 2021) after the 2nd dose,
- Be fully effective in as short as 7 days after the 2nd dose,
- Cause reactions that are mild, and short-lived, and
- Work against the Delta variant.
Data Limitations:
- The trial, given participation size of 1,528, cannot reveal rarer adverse reactions, such as those occuring in 1 per 10,000 or more participants, etc. The data is estimated to be safe based on data from age 16-25 group.
Potential reasons for a vaccination as soon as possible:
- Your child has a significant medical condition that are risk factors for a severe case of COVID-19: asthma, neurological conditions, heart disease, etc.
- Your child will attend a large gathering this holiday season with others outside the immediate household.
- Your child lives with at-risk relatives: age >65, immunocompromised, significant medical history
- Your child is regularly exposed to SARS-CoV-2 (such as regular interaction with adults and children outside the immediate household)
Discuss with your physician to see if the vaccination is right for your child.